Detecting antibodies with glowing proteins, thread and a smartphone
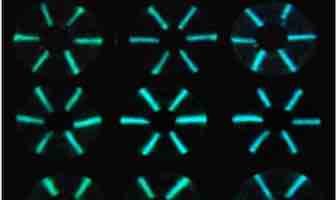 To defend the body, the immune system makes proteins known as antibodies that latch onto the perceived threat, be it HIV, the new coronavirus or, as is the case in autoimmune disease, part of the body itself. In a new proof-of-concept study in ACS Sensors, researchers describe a new system for detecting antibodies within a pinprick of blood within minutes, using an unlikely combination of cotton thread, glowing proteins and a smartphone camera.
While some tests simply detect the presence of an antibody, sometimes doctors want to know how much is circulating in the blood. Such quantitative tests are used to diagnose a number of conditions, including infections and autoimmune diseases. Although a quantitative antibody test is not yet approved for use in the U.S., such a test could potentially aid in assessing immunity to SARS-CoV-2. However, quantitative testing currently requires expensive, sophisticated instruments in labs, and efforts to make it more accessible have had only limited success. So, Maarten Merkx, Daniel Citterio and colleagues tested an approach that could provide a small, inexpensive alternative.
To defend the body, the immune system makes proteins known as antibodies that latch onto the perceived threat, be it HIV, the new coronavirus or, as is the case in autoimmune disease, part of the body itself. In a new proof-of-concept study in ACS Sensors, researchers describe a new system for detecting antibodies within a pinprick of blood within minutes, using an unlikely combination of cotton thread, glowing proteins and a smartphone camera.
While some tests simply detect the presence of an antibody, sometimes doctors want to know how much is circulating in the blood. Such quantitative tests are used to diagnose a number of conditions, including infections and autoimmune diseases. Although a quantitative antibody test is not yet approved for use in the U.S., such a test could potentially aid in assessing immunity to SARS-CoV-2. However, quantitative testing currently requires expensive, sophisticated instruments in labs, and efforts to make it more accessible have had only limited success. So, Maarten Merkx, Daniel Citterio and colleagues tested an approach that could provide a small, inexpensive alternative.
- Monday, June 22, 2020



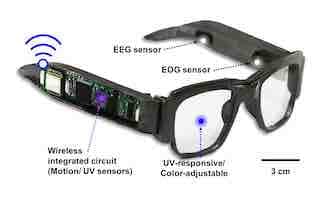 Fitness tracker bracelets and watches provide useful information, such as step count and heart rate, but they usually can’t provide more detailed data about the wearer’s health. Now, researchers reporting in ACS Applied Materials & Interfaces have developed smart electronic glasses (e-glasses) that not only monitor a person’s brain waves and body movements, but also can function as sunglasses and allow users to control a video game with eye motions.
Devices that measure electrical signals from the brain (electroencephalogram; EEG) or eyes (electrooculogram; EOG) can help diagnose conditions like epilepsy and sleep disorders, as well as control computers in human-machine interfaces. But obtaining these measurements requires a steady physical contact between skin and sensor, which is difficult with rigid devices. Suk-Won Hwang and colleagues wanted to integrate soft, conductive electrodes into e-glasses that could wirelessly monitor EEG and EOG signals, ultraviolet (UV) intensity, and body movements or postures, while also acting as a human-machine interface.
Fitness tracker bracelets and watches provide useful information, such as step count and heart rate, but they usually can’t provide more detailed data about the wearer’s health. Now, researchers reporting in ACS Applied Materials & Interfaces have developed smart electronic glasses (e-glasses) that not only monitor a person’s brain waves and body movements, but also can function as sunglasses and allow users to control a video game with eye motions.
Devices that measure electrical signals from the brain (electroencephalogram; EEG) or eyes (electrooculogram; EOG) can help diagnose conditions like epilepsy and sleep disorders, as well as control computers in human-machine interfaces. But obtaining these measurements requires a steady physical contact between skin and sensor, which is difficult with rigid devices. Suk-Won Hwang and colleagues wanted to integrate soft, conductive electrodes into e-glasses that could wirelessly monitor EEG and EOG signals, ultraviolet (UV) intensity, and body movements or postures, while also acting as a human-machine interface.
 WASHINGTON — Antiviral drugs could help us fight the new coronavirus, but currently, we don’t have a highly potent, effective antiviral that cures COVID-19. Why not? We called a few virologists to find out:
WASHINGTON — Antiviral drugs could help us fight the new coronavirus, but currently, we don’t have a highly potent, effective antiviral that cures COVID-19. Why not? We called a few virologists to find out:
 WASHINGTON — As demand for higher-efficiency and smaller electronics grows, so does demand for a new generation of materials that can be printed at ever smaller dimensions. Such materials are critical to national security applications and space exploration. But materials that work well on Earth don’t always hold up well at high altitudes and in space. Scientists are now creating new metal-based nanomaterials for circuit boards that could be resistant to the high-altitude radiation encountered by electronics in aerospace equipment, fighter jets and weapon systems.
WASHINGTON — As demand for higher-efficiency and smaller electronics grows, so does demand for a new generation of materials that can be printed at ever smaller dimensions. Such materials are critical to national security applications and space exploration. But materials that work well on Earth don’t always hold up well at high altitudes and in space. Scientists are now creating new metal-based nanomaterials for circuit boards that could be resistant to the high-altitude radiation encountered by electronics in aerospace equipment, fighter jets and weapon systems.
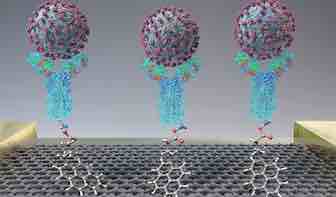 According to many experts, early diagnosis and management are critical for slowing the spread of SARS-CoV-2, the new coronavirus that causes COVID-19. Therefore, the race is on to develop diagnostic tests for the virus that are faster, easier and more accurate than existing ones. Now, researchers reporting in ACS Nano have developed a field-effect transistor-based biosensor that detects SARS-CoV-2 in nasopharyngeal swabs from patients with COVID-19, in less than one minute.
According to many experts, early diagnosis and management are critical for slowing the spread of SARS-CoV-2, the new coronavirus that causes COVID-19. Therefore, the race is on to develop diagnostic tests for the virus that are faster, easier and more accurate than existing ones. Now, researchers reporting in ACS Nano have developed a field-effect transistor-based biosensor that detects SARS-CoV-2 in nasopharyngeal swabs from patients with COVID-19, in less than one minute.
 When a dead body is found, one of the first things a forensic pathologist tries to do is estimate the time of death. There are several ways to do this, including measuring body temperature or observing insect activity, but these methods don’t always work for corpses found in water. Now, researchers are reporting a mouse study in ACS’ Journal of Proteome Research showing that certain proteins in bones could be used for this determination.
When a dead body is found, one of the first things a forensic pathologist tries to do is estimate the time of death. There are several ways to do this, including measuring body temperature or observing insect activity, but these methods don’t always work for corpses found in water. Now, researchers are reporting a mouse study in ACS’ Journal of Proteome Research showing that certain proteins in bones could be used for this determination.
 Over 80% of chemicals used to make pharmaceuticals sold in Europe originate from China or India, according to the European Fine Chemicals Group. When COVID-19 emerged in Wuhan and spread across the globe, experts worried about disruption of the drug supply chain. Now, nations are rethinking their dependence on other countries for pharmaceutical ingredients and finished drugs, according to an article in Chemical & Engineering News (C&EN), the weekly newsmagazine of the American Chemical Society.
Over 80% of chemicals used to make pharmaceuticals sold in Europe originate from China or India, according to the European Fine Chemicals Group. When COVID-19 emerged in Wuhan and spread across the globe, experts worried about disruption of the drug supply chain. Now, nations are rethinking their dependence on other countries for pharmaceutical ingredients and finished drugs, according to an article in Chemical & Engineering News (C&EN), the weekly newsmagazine of the American Chemical Society.
 Gluten is enemy No. 1 for those with celiac disease, and it’s hard to avoid. Episodes of this chronic autoimmune illness can be triggered by ingesting gluten, a key protein in wheat and some other grains. Researchers have been exploring how gut bacteria, especially Bifidobacteria, could be used as a treatment. Now, scientists publishing the results of laboratory experiments in ACS’ Journal of Agricultural and Food Chemistry report how specific types of Bifidobacteria work.
Gluten is enemy No. 1 for those with celiac disease, and it’s hard to avoid. Episodes of this chronic autoimmune illness can be triggered by ingesting gluten, a key protein in wheat and some other grains. Researchers have been exploring how gut bacteria, especially Bifidobacteria, could be used as a treatment. Now, scientists publishing the results of laboratory experiments in ACS’ Journal of Agricultural and Food Chemistry report how specific types of Bifidobacteria work.
 Patients with Alzheimer’s disease (AD) are often prescribed drugs for other conditions — including diabetes or high blood pressure — at the same doses as those without dementia. That practice might need to be reexamined in the wake of new mouse studies reported in ACS’ Molecular Pharmaceutics. The findings suggest that AD could alter absorption of medications from the digestive tract, so dosages might need to be adjusted for these patients.
Patients with Alzheimer’s disease (AD) are often prescribed drugs for other conditions — including diabetes or high blood pressure — at the same doses as those without dementia. That practice might need to be reexamined in the wake of new mouse studies reported in ACS’ Molecular Pharmaceutics. The findings suggest that AD could alter absorption of medications from the digestive tract, so dosages might need to be adjusted for these patients.