Interestingly, in recent years several derivatives of thalidomide have received approval for treatment of certain cancers, both in the U.S. and in Europe
Placeboism

Everyone knows (or ought to): Everything is getting better all the time. That’s certainly true in spring or early summer, when nature re-awakens after a long and cold winter but, just perhaps, not all the time.
And that’s why cunning politicians like to make big promises and have elections at those times. That’s just one example of the placebo effect, the proclamation that “your vote counts.”
But there are ever better reasons for placebos:
The Placebo Effect
The placebo effect is real. Many studies have confirmed its existence. For some people, just taking any “medicine” will make them feel better; Wiki confirms it Who could argue with that?
After all, just taking this novel XYZ-product is promising some benefit. So what harm could it be to give it a try?
For some, the mere mention of hope produces a beneficial effect, a more positive and life-embracing attitude. There’s nothing wrong with that thinking, as long is the expectations are within the framework of cautions, like ineffectiveness and/or potentially undesirable “side effects” of an experimental stage novel drug. Those patients are often part of a so-called ”double blind study.”
Double Blind Studies
When it comes to field testing new materials (for treatment against whatever affliction or disease), and after all other initial studies to ascertain a lack of detrimental effects have been done, a new drug candidate is being subjected to a “double blind” study of its efficacy – with real people – in real hospitals, etc.
That’s when the chaff is separated from the wheat.
In a Double Blind Study (DBS), neither the prescribing physician nor the novel-drug-candidate-consuming patient know “what, exactly” they are prescribing, respectively consuming. Therefore, it’s impossible to fake the “right” answer. As a result, the patient is left to his/her own true assessment as to whether they feel better or not. Such DBS are also termed “Randomized controlled trial”, though that term has been replaced with new terminologies that are better defined as to who knows what or not.
Many new drug candidates fail in such tests – they are not significantly different from true placebos. It certainly is disappointing for the patients that hoped for a cure, for the company that developed the product, and its shareholders. That’s why smaller pharmaceutical drug developers’ shares can sharply rise or fall with the outcomes of such DBS tests.
Also, that’s why developing novel drugs has become such an expensive enterprise and any effective medication naturally demands its corresponding price. Of course, comparatively small size molecules can generally be synthesized with a good yield, in almost any laboratory. The cost is not in the substance itself but the extensive testing requirements that can (and often do) take years to complete. How critical such testing principles are can be demonstrated on one sad example: the thalidomide case.
The Thalidomide Case
Thalidomide was developed and marketed in the mid 1950’s as a sedative and to relieve relatively minor ailments, such as "anxiety, insomnia, gastritis, and tension." It was also found to relieve morning sickness in expecting mothers and widely prescribed for that purpose. That use turned into a major disaster. Many women who took that drug during pregnancy gave birth to severely deformed offspring, most commonly with massive malformation of the limbs.
The cause-effect relationship between consumption of this drug and appearance of malformed infants took some time to be recognized.
Thalidomide is a really small molecule (molecular weight 258) and simple structure. However, it has one carbon atom that is “chiral”, meaning its four bonds extending in tetrahedral fashion from the central carbon atom center) can exist in a “right” (R) or “left” (S) configuration. Only one of these two enantiomers (mirror image-like) is actually teratogenic (causing or foster malformation of an embryo); the other one is benign.
The nearby graphs show that with 3D information (modified from Thalidomide):
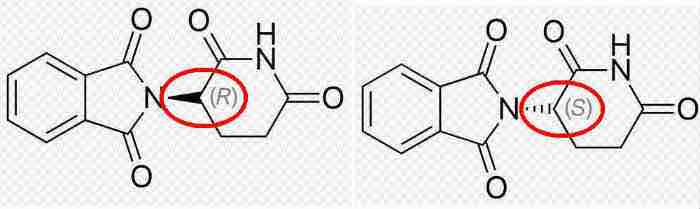
Fig.: The two enantiomers of thalidomide; the red circles mark the chiral carbon atom, also indicated by the solid and dashed wedges emanating from it. Only the S-form (at bottom) is bioactive.
Except for this minor geometric difference between the R and S configurations, both molecules are identical. However, their biological properties are miles apart. Moreover, even pure samples of the non-teratogenic compound (upper part in the figure) are converted in a placenta into a mixture of both forms of the material. That conversion was not (or could not be – I’m unsure of that) recognized with the standard testing procedures of the time; remember, that was six decades ago. In any event, many countries instituted more stringent testing requirements following this unfortunate situation.
Interestingly, in recent years several derivatives of thalidomide have received approval for treatment of certain cancers, both in the U.S. and in Europe.
Dr. Klaus L.E. Kaiser -- Bio and
Archives |
Comments
Dr. Klaus L.E. Kaiser is author of CONVENIENT MYTHS, the green revolution – perceptions, politics, and facts Convenient Myths

 Everyone knows (or ought to): Everything is getting better all the time. That’s certainly true in spring or early summer, when nature re-awakens after a long and cold winter but, just perhaps, not all the time.
And that’s why cunning politicians like to make big promises and have elections at those times. That’s just one example of the placebo effect, the proclamation that “your vote counts.”
Everyone knows (or ought to): Everything is getting better all the time. That’s certainly true in spring or early summer, when nature re-awakens after a long and cold winter but, just perhaps, not all the time.
And that’s why cunning politicians like to make big promises and have elections at those times. That’s just one example of the placebo effect, the proclamation that “your vote counts.” Fig.: The two enantiomers of thalidomide; the red circles mark the chiral carbon atom, also indicated by the solid and dashed wedges emanating from it. Only the S-form (at bottom) is bioactive.
Except for this minor geometric difference between the R and S configurations, both molecules are identical. However, their biological properties are miles apart. Moreover, even pure samples of the non-teratogenic compound (upper part in the figure) are converted in a placenta into a mixture of both forms of the material. That conversion was not (or could not be – I’m unsure of that) recognized with the standard testing procedures of the time; remember, that was six decades ago. In any event, many countries instituted more stringent testing requirements following this unfortunate situation.
Interestingly, in recent years several derivatives of thalidomide have received approval for treatment of certain cancers, both in the U.S. and in Europe.
Fig.: The two enantiomers of thalidomide; the red circles mark the chiral carbon atom, also indicated by the solid and dashed wedges emanating from it. Only the S-form (at bottom) is bioactive.
Except for this minor geometric difference between the R and S configurations, both molecules are identical. However, their biological properties are miles apart. Moreover, even pure samples of the non-teratogenic compound (upper part in the figure) are converted in a placenta into a mixture of both forms of the material. That conversion was not (or could not be – I’m unsure of that) recognized with the standard testing procedures of the time; remember, that was six decades ago. In any event, many countries instituted more stringent testing requirements following this unfortunate situation.
Interestingly, in recent years several derivatives of thalidomide have received approval for treatment of certain cancers, both in the U.S. and in Europe.